Background: Therapies for relapsed or refractory (R/R) AML remain limited, with low response rates and short duration of response, especially in patients ineligible for intensive cytotoxic chemotherapy. Combinations of venetoclax (VEN), chidamide (CHI) plus azacytidine (AZA) have shown to be synergistic with low intensity and intensive chemotherapy in preclinical data and in relapsed or refractory (R/R) setting (Phase II, 2022 ASH). Here we present updated results of this Phase II trial.
Methods: Eligible patients were >18 yrs old with R/R AML, defined as lack of response rate after at least one cycle of induction or relapsed disease with any prior number of treatments. Patients received AZA 100mg on D1-7, VEN 200mg on D1-14, and CHI 30mg biweekly in 28-day cycles. The primary objective was evaluation of CR/CRi. Secondary objectives included event-free survival (EFS), overall survival (OS) and adverse events (AEs). Clinical responses were determined using the 2017 ELN criteria.
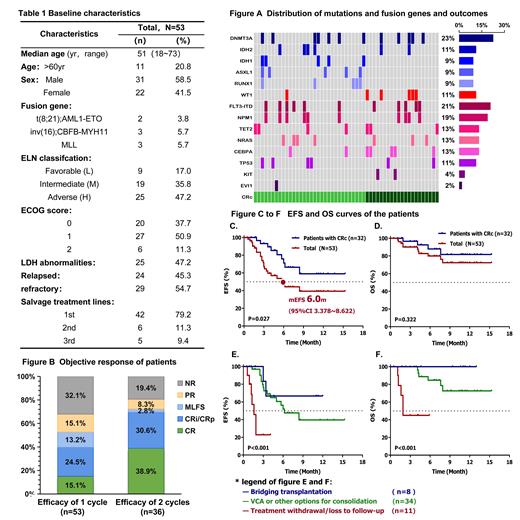
Results: Between January 2022 and April 2023, 53 patients with R/R AML were enrolled in the study. The median age was 51 years (range 18-73). This regimen was salvage 1 in 79% (42) of patients, salvage 2 in 11% (6) and salvage 3 in 9% (5). 17%, 36%, and 47% of patients were classified as favorable, intermediate, and adverse ELN risk, respectively ( Table 1). 11% of patients had TP53 mutation, 22% had DNMT3A mutation, 21% had FLT3-ITD mutation, 9% had ASXL1 mutation, 19% had NPM1 mutation, 9% had IDH1 mutation, 11% had IDH2 mutation ( Fig. A).
Among the 53 patients, the ORR of these patients was 68% with CRc rate of 53% after one cycle, and 81% with CRc rate of 72% after two cycles ( Fig. B). By ELN risk, ORR and CRc rates were 70% and 50% for favorable risk, 40% and 40% for intermediate risk, and 43% and 30% for adverse risk. Pretreatment IDH1 and IDH2 mutations were associated with higher CRc rates (75% and 50%, respectively). The CRc rate in patients with TP53 mutations was 67%, with response noted among patients with concurrent IDH1/ IDH2 mutations.
With a median follow-up of 6.7 months, the median EFS and OS were 6 (95%CI 3.3-8.6) months and not reached (NR), respectively. Among patients with CRc, the 6-month EFS and OS were 64% and 88%, respectively, among those receiving this regimen as first salvage had encouraging outcomes (median EFS, NR) ( Fig. C-D). Of the 8 patients that proceeding with allogeneic hematopoietic stem cell transplant (allo-HSCT), the median EFS and OS were not reached after transplant ( Fig. E-F)
No non-hematologic AEs of grade ≥3 were observed. The most common grade 1-2 non-hematologic AEs included nausea (28%), fatigue (28%), hypokalemia (17%), hypoproteinemia (17%), elevated transaminase levels (33%) and hyperbilirubinemia (11%). The occurrence rates of grade 3/4 hematologic adverse events (AEs) were as follows: white blood cell decrease (87%), median time to white blood cell recovery 23 (range 4-29) days; neutropenia (96%), neutropenia with fever (17%), median time to neutrophil recovery 25 (range 10-29) days; platelet decrease (68%), median time to platelet recovery 28.5 (range 7-44) days; anemia (64%), median time to hemoglobin recovery 29 (range 13-56) days. 30-day and 60-day mortality were 0% and 5.7%, respectively. 3 deaths occurred within 60 days in patients with persistent leukemia.
Conclusions: Thecombination of VEN and CHI plus AZA (VCA) demonstrated manageable safety and encouraging efficacy in patients with R/R AML. IDH1/ IDH2 mutations were associated with the sensitivity of VCA, even in some patients with concurrent TP53 mutations.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal